Goch, Germany (5th July 2022) - DFE Pharma, a global leader in pharma- and nutraceutical excipient solutions, announced today the expansion of its Dry Powder Inhalation (DPI) portfolio with the launch of Lactohale® 400.
The addition of Lactohale® 400 further enhances the completeness of DFE Pharma’s portfolio of inhalation grade lactose. As the whole Lactohale® brand, this new grade can be customized to meet any specific need of pharmaceutical companies and can be developed jointly with formulators to ensure exactly the right outcomes for patients.
The comprehensive range of lactose-based excipients by DFE Pharma, is designed to offer the right characteristics for the full variety of inhaled medicines in the market. For those therapies in development, DFE Pharma works together with R&D teams of pharmaceutical companies, to find the most appropriate formulation for each molecule and device combination.
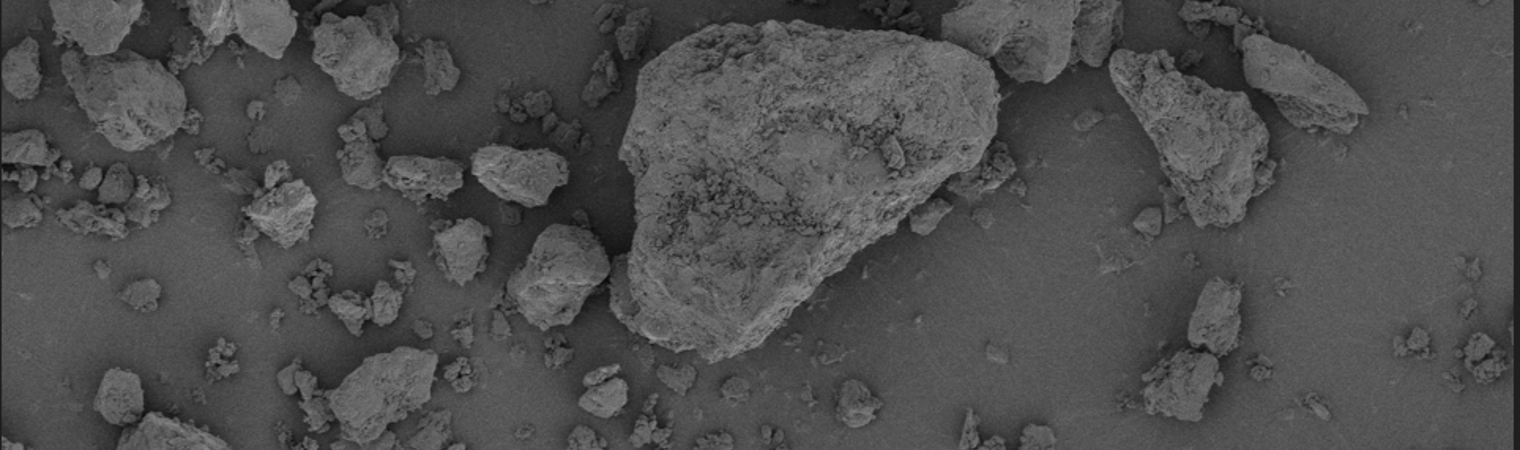
Lactohale® 400 is a milled, anhydrous lactose grade, that can be used in DPI applications where a relatively good flow with some cohesiveness is required. The anhydrous nature of Lactohale® 400 differentiates it from the most commonly used lactose carriers in DPI formulations. It can provide a different aerosolization performance than lactose monohydrate with the same particle size.
“As codevelopers of Dry Powder Inhalation (DPI), DFE Pharma has a long heritage and great expertise in excipients for inhalation, while we continuously innovate in new premium solutions for inhaled therapies,” said Martti Hedman, CEO of DFE Pharma, “With Lactohale® 400, DFE Pharma expands its comprehensive lactose-based portfolio, designed to offer optimized excipients to match the increasing range of inhalant devices, each with different performance characteristics”.