Additionally, BioHale® Sucrose is preferred in applications where solubility or viscosity issues are of supreme importance. BioHale® Sucrose is well suited to provide solution-state stabilization, as well as cryo- and lyo-protection for therapeutic proteins as excipients in the formulations. Sucrose can generally be considered as suitable in most cases, unless constrained by a low-pH formulation.
BioHale® Sucrose can be used in various administration forms, such as for parenteral, oral or ophthalmic route. Beyond our excipient portfolio, customers benefit from our technical solutions and regulatory support to unlock their competitive advantage. Our BioHale® excipients comply with the global regulatory requirements of the pharmaceutical industry (Ph. Eur., USP-NF, JP, ChP) and hold a Chinese Drug Master File (cDMF).
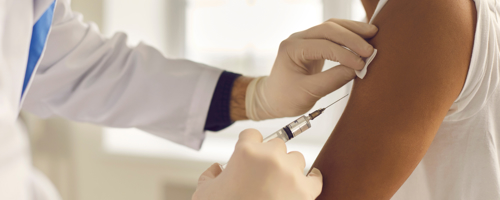
With BioHale® Sucrose, we are currently supporting COVID-19 vaccine production around the world. Read more: DFE Pharma Proudly Supports COVID-19 Vaccination Supply in Pakistan
If you would like to meet our Biopharma expert, please click here.