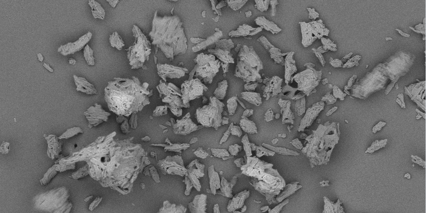
Pharmacel® 112 is a directly compressible microcrystalline cellulose grade with a low moisture content (LOD Max. 1.5% w/w), especially designed for formulating active pharmaceutical ingredients (APIs) with a high sensitivity for moisture.
This website uses cookies to ensure you get the best experience on our website. Cookies
Cookies to store necessary information
| Name | Provider | Purpose | Expiry | Type |
|---|---|---|---|---|
| .AspNetCore.Mvc.CookieTempDataProvider | dfepharma.com | Preserves the visitor's session state across page requests. | Session | HTTP |
| .AspNetCore.Antiforgery | dfepharma.com | Helps prevent Cross-Site Request Forgery (CSRF) attacks. | Session | HTTP |
| formulation-tool-send-advice-to-yourself | dfepharma.com | To process a send advice to yourself request. | Session | HTTP |
| formulation-tool-request-sample | dfepharma.com | To process a sample request. | Session | HTTP |
| UMB_SESSION | dfepharma.com | Preserves the visitor's session state across page requests. | Session | HTTP |
| ARRAffinitySameSite | dfepharma.com | Used to distribute traffic to the website on several servers in order to optimise response times. | Session | HTTP |
| AWSALBCORS | rocketfuel.humanfirstdigital.agency | Registers a unique ID that is used to generate statistical data on how the visitor uses the website. | One week | HTTP |
| quote-form | dfepharma.com | To process a quote request. | One week | HTTP |
| request-sample | dfepharma.com | To process a sample request. | One week | HTTP |
Cookies are small text files that can be used by websites to make a user's experience more efficient.
The law states that we can store cookies on your device if they are strictly necessary for the operation of this site. For all other types of cookies we need your permission.
This site uses different types of cookies. Some cookies are placed by third party services that appear on our pages.
You can at any time change or withdraw your consent from the Cookie Declaration on our website.
Learn more about who we are, how you can contact us and how we process personal data in our Privacy Policy.